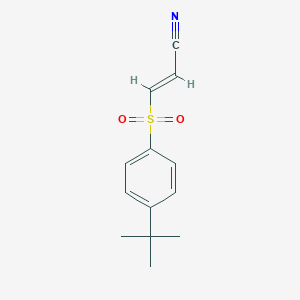Difference between revisions of "Bay11-7085"
(→Reference) |
|||
| (14 intermediate revisions by the same user not shown) | |||
| Line 2: | Line 2: | ||
| __TOC__ | | __TOC__ | ||
|} | |} | ||
| − | {{#invoke:InfoboxforLigand|run|196309-76-9|C13H15NO2S|249.33 g/mol|[https://pubchem.ncbi.nlm.nih.gov/compound/5353432 5353432]|Synthetic|Carbon-Sulfur Bond: Michael addition}} | + | {{#invoke:InfoboxforLigand|run|196309-76-9|C13H15NO2S|249.33 g/mol|[https://pubchem.ncbi.nlm.nih.gov/compound/5353432 5353432]|[[:Category:Synthetic|Synthetic]]|[[:Category:Michael addition|Carbon-Sulfur Bond: Michael addition]]}} |
==Molecular Structure== | ==Molecular Structure== | ||
| Line 20: | Line 20: | ||
:Unknown | :Unknown | ||
| − | == | + | ==Activity== |
| − | {| class="wikitable" | + | :{| class="wikitable" |
| − | ! | + | ! |
! PTP1B (Homo sapiens) - P18031 | ! PTP1B (Homo sapiens) - P18031 | ||
! TCPTP (Homo sapiens) - P17706 | ! TCPTP (Homo sapiens) - P17706 | ||
| Line 44: | Line 44: | ||
==Reference== | ==Reference== | ||
| − | # Krishnan | + | # Krishnan N, Bencze G, Cohen P, et al. '''The anti-inflammatory compound BAY-11-7082 is a potent inhibitor of protein tyrosine phosphatases[J].''' The FEBS journal, 2013, 280(12): 2830-2841. [https://www.ncbi.nlm.nih.gov/pubmed/?term=23578302 23578302]<br/> |
| − | # Pierce | + | # Pierce J W, Schoenleber R, Jesmok G, et al. '''Novel inhibitors of cytokine-induced IκBα phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo[J].''' Journal of Biological Chemistry, 1997, 272(34): 21096-21103. [https://www.ncbi.nlm.nih.gov/pubmed/?term=9261113 9261113]<br/> |
| − | [[Category: | + | [[Category:Ligands]] |
| − | [[Category: | + | [[Category:Tyrosine-protein phosphatase non-receptor type 1]] |
| + | [[Category:Synthetic]] | ||
| + | [[Category:Michael addition]] | ||
Latest revision as of 23:29, 19 August 2019
| Basic Information | |
|---|---|
| CAS Number | 196309-76-9 |
| Molecular Formula | C13H15NO2S |
| Molecular Weight | 249.33 g/mol |
| PubChem CID | 5353432 |
| Type | Synthetic |
| Bond Type | Carbon-Sulfur Bond: Michael addition |
Molecular Structure
Name and Identifier
- Chemical Name/Synonyms
- 3-((4-(1,1-dimethylethyl)phenyl)sulfonyl)-2-propenenitrile
- (E)-3-(4-Tert-butylphenylsulfonyl)acrylonitrile
- SMILES
- CC(C)(C)C1=CC=C(C=C1)S(=O)(=O)C=CC#N
Cysteinome Target
Related PDB
- Unknown
Activity
PTP1B (Homo sapiens) - P18031 TCPTP (Homo sapiens) - P17706 IKK-alpha (Homo sapiens) - O15111 Experimental data Ki: 36 µM.
LC-MS/MSKi: 39 µM.
LC-MS/MSIC50: 5 µM PubMed ID 23578302 23578302 9261113 Cys site C215 C216 C178
Reference
- Krishnan N, Bencze G, Cohen P, et al. The anti-inflammatory compound BAY-11-7082 is a potent inhibitor of protein tyrosine phosphatases[J]. The FEBS journal, 2013, 280(12): 2830-2841. 23578302
- Pierce J W, Schoenleber R, Jesmok G, et al. Novel inhibitors of cytokine-induced IκBα phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo[J]. Journal of Biological Chemistry, 1997, 272(34): 21096-21103. 9261113